Answer
55.9 g/mol
Step-by-step explanation
Given:
The mass of the sample = 1.95 g
Moles of Cl in the sample = 0.03606 mol
What to find:
To determine the atomic mass of M.
Step-by-step solution:
Step 1: Determine the mass of Cl in the sample.
Since it was given that 0.03606 mol of Cl is found in the sample, then the mass of Cl in the 1.95 g of the sample will be:
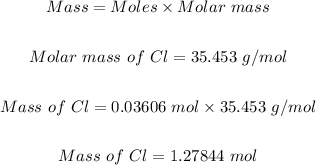
Step 2: Determine the mass of metal M in the compound.
Mass of metal M = Mass of MCl₃ sample - Mass of Cl
Mass of metal M = 1.95 g - 1.27844 g
Mass of metal M = 0.67156 g
Step 3: Determine the mole of M.
From the formula of the compound, (MCl₃), the M to Cl molar ratio is 1 to 3. Therefore, moles M in the sample:
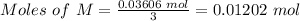
Step 3: Calculate the atomic mass of M.
The atomic mass of M can be calculated using the mass of M in step 2 and and the moles of M in step 3 above:
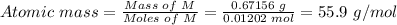
Hence, the atomic mass of M is 55.9 g/mol