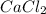
dissolves in water to give
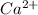
and
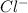
ions according to the following reaction:
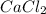
-------->
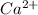
+ 2
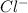
So, according to the above reaction, 1 mole of
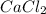
produce 2 moles of
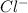
ion,
So, 0.3 mole will give = 0.3 x 2 = 0.6 moles of
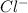
ion
So, Molar concentration =
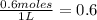
Note: 1L = 1000mL