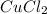Answer : The chemical formula will be,
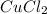
Explanation :
According to the question, the
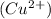 ion reacted with the chloride ion and the chloride ion is the counter ion in the chemical formula.
ion reacted with the chloride ion and the chloride ion is the counter ion in the chemical formula.
As we know that the copper ion is a cation (positively charged ion) and copper has ability to lose 2 electrons easily to achieve the stability.
And the counter ion is the chloride ion which is an anion (negatively charged ion) and chloride ion has ability to gain electrons to achieve the stability. The chloride ion is written as,
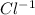 that means it has (-1) charge.
that means it has (-1) charge.
From this we conclude that to maintain the electricalneutrality, two chloride ion will react with the 1 copper ion.
Thus, the chemical formula becomes,