The simple trick which one can consider in such problem where it is asked for positron emission is :
When the atomic number goes DOWN by one and mass number remains unchanged, then a positron is emitted.
a.
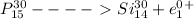
Here the atomic number decreases by one.
Similarly, options b and d are eliminated.
Option c is also not the answer.
For c, Count the atomic number on left side and compare it with right side. You will see it is 9 on left and 8 on right. Atomic no. did go down by 1. But the atomic mass is changed as well.