Answer:
If the concentration of b is increased by a factor of 2.00, the rate will be increased four times.
Step-by-step explanation:
This happens because the concentration of compound b is squared. This means that if the concentration doubles (2*b), by placing it in the equation, the coefficient will be squared, leaving 4*
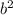 .
.
In this way, the rate will increase 4 times concerning the original one.
rate1 = k[a][b]^2
rate2 = k[a][2*b]^2
rate2 = k[a]*4*[b]^2
rate2 = 4 * k[a][b]^2
rate2 = 4 * rate 1
Have a nice day!