Answer:
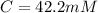
Step-by-step explanation:
Hello,
By using the ideal gas equation, one could find the molar solubility for the given pressure as shown below:
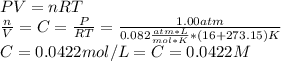
Finally, as it is required in millimolar units, mM, one applies the following conversion:
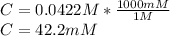
Best regards.