Answer: The average mass of the oxygen atom in these meteorites will be greater than the terrestrial oxygen atom.
Step-by-step explanation:
Average atomic mass of an element is defined as the summation of the product of the atomic masses and fractional abundances of its isotopes.
Equation to calculate the average atomic mass will be given by:
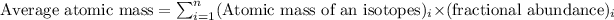
We are given that the abundance ratio of the oxygen in meteorites is more than the terrestrial oxygen atom.
As, from the formula, average atomic mass of an element is directly proportional to the fractional abundance of the isotopes. Hence, with increase in the abundance, the average atomic mass also increases.
Therefore, the average mass of the oxygen atom in these meteorites will be greater than the terrestrial oxygen atom.