The number of moles of NaCl required to form 5M of 450 ml solution is,
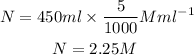
The mass of the NaCl is,
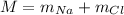
Substituting the known values,
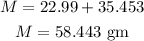
Thus, the amount of NaCl required in gm to make the given solution is,
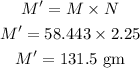
Hence, the amount of NaCl equired for the given solution is 131.5 gm