ANSWER
A. The system did 2000 J of work on the environment
Step-by-step explanation
We want to identify what accounts for the missing 2000 J of energy.
According to the first law of thermodynamics, the change in the internal energy of a system is equal to the difference between the heat added to the system and the work done by the system:
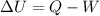
From the given information, we see that:
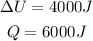
Solving for W:
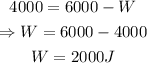
Since the work done is positive, it implies that the 2000 J accounts for the work that the system did on the environment.
Thecorrect answer is option A.