Answer:
Two options: vanadium (III) chlorate or vanadic chlorate.
Step-by-step explanation:
Hello,
In this case, V accounts for vanadium, Cl for chlorine and O for oxygen. Now, as you've got v(clo3)3, one should write it with the proper capitalization:
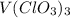
In such a way, the name depends on the oxidation states of both vanadium and chlorine. At first, one realizes that vanadium has +3 as the oxidation state as all the oxyanions formed by chlorine have -1 as the total charge. Now, the anion
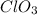 suggests that the chlorine has +5 as the oxidation state for it to has a total oxidation state of -1, in such a way, by following the IUPAC rules, one states is name as vanadium (III) chlorate or vanadous chloride. For the spelling, procure to respect spaces and to use lower cases for the resulting names.
suggests that the chlorine has +5 as the oxidation state for it to has a total oxidation state of -1, in such a way, by following the IUPAC rules, one states is name as vanadium (III) chlorate or vanadous chloride. For the spelling, procure to respect spaces and to use lower cases for the resulting names.
Best regards.