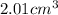Answer : The volume of a piece of metal is
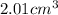
Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used for density :
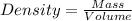
Given :
Mass of a piece of metal = 9.37 grams
Density of metal =
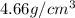
Now put all the given values in the above formula, we get the volume of a piece of metal.
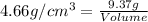
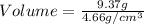
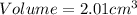
Therefore, the volume of a piece of metal is