Step-by-step explanation:
Atomic number of an atom means the sum of total number of protons present in an atom.
Mass number is the sum of total number of protons and neutrons present in an atom.
And, when we write the symbol of an atom then it is represented like this
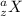 , where "a" represents the mass number of the atom and "z" represents the number of protons.
, where "a" represents the mass number of the atom and "z" represents the number of protons.
Therefore, in plutonium-239 it is shown that mass number is 239 and atomic number of a plutonium atom is 94. Now, calculate its number of neutrons as follows.
Mass number = no. of protons + no. of neutrons
239 = 94 + no. of neutrons
no. of neutrons = 239 - 94
= 145
Thus, we can conclude that in plutonium-239 number of protons is 94, number of neutrons is 145 and its symbol is
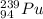 .
.