Answer:
Carbon and carbon tetrahydride (methane).
Step-by-step explanation:
Hello,
In this case, it is not necessary to compute the particles for all the given masses, it is enough by knowing each substance's moles by knowing their molar mass and subsequently proof they equals 1 mole as long as 1 mole equals 6.022x10²³ which is the Avogadro's number; in such a way, the molar masses are:
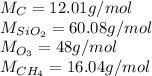
Therefore, the two cases are carbon and carbon tetrahydride or methane as shown below:
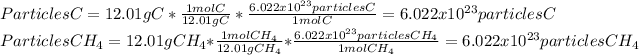
Best regards.