Answer: The
 for the reaction is -382 kJ.
for the reaction is -382 kJ.
Step-by-step explanation:
For the following reaction:
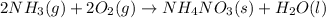
- Equation used to calculate
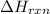 is:
is:
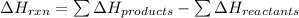
We are given:
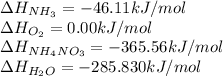
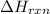 for the reaction is calculated by:
for the reaction is calculated by:
![\Delta H_(rxn)=[1(\Delta H_(NH_4NO_3))+1(\Delta H_(H_2O))]-[2(\Delta H_(NH_3))+2(\Delta H_(O_2))]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/zvict73jzyq7hdt0fficu6wztcztwt9x8v.png)
Putting values in above equation, we get:
![\Delta H_(rxn)=[1(-365.56)+1(-285.83)]-[2(-46.11)+2(0)]kJ\\\\\Delta H_(rxn)=-559.17kJ=559170J](https://img.qammunity.org/2018/formulas/chemistry/middle-school/17nxixxpmq9qoj82g28czezmamlwhxkcs0.png)
- Equation used to calculate
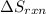 is:
is:
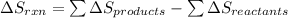
We are given:
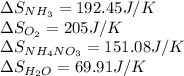
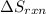 for the reaction is calculated by:
for the reaction is calculated by:
![\Delta S_(rxn)=[1(\Delta S_(NH_4NO_3))+1(\Delta S_(H_2O))]-[2(\Delta S_(NH_3))+2(\Delta S_(O_2))]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/2x8dmu0qupirqlyqt3vckhucjlmobxafow.png)
Putting values in above equation, we get:
![\Delta S_(rxn)=[1(151.08)+1(69.91)]-[2(192.45)+2(205)]J/K\\\\\Delta S_(rxn)=-573.91J/K](https://img.qammunity.org/2018/formulas/chemistry/middle-school/9qw45qzh6ta0fxq5bvl2xetddwrf16htkt.png)
- Now, to calculate
 , the equation used is:
, the equation used is:
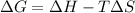
We are given:
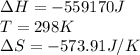
Putting values in above equation, we get:
![\Delta G=(-559170J)-[298K* (-573.91J/K)]\\\\\Delta G=-382kJ](https://img.qammunity.org/2018/formulas/chemistry/middle-school/6mors87jy30f6krvgjtowtcbk69weroipz.png)
Hence, the
 for the reaction is -382 kJ.
for the reaction is -382 kJ.