Step-by-step explanation:
The given chemical equation is as follows.
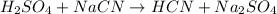
Number of reactant atoms are as follows.
- H = 2
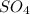 = 1
= 1- Na = 1
- CN = 1
Number of product atoms are as follows.
- H = 1
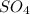 = 1
= 1- Na = 1
- CN = 1
Thus, in order to balance the equation, multiply NaCN by 2 on the reactant side and multiply HCN by 2 on the product side. Therefore, the balanced chemical equation will be as follows.
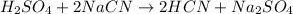
Hence, we need
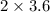 = 7.2 moles of NaCN to react completely with 3.6 moles of
= 7.2 moles of NaCN to react completely with 3.6 moles of
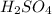 .
.