Answer: negative enthalpy change and positive entropy change
Explanation:-
According to Gibb's equation:
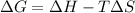
 = Gibbs free energy
= Gibbs free energy
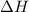 = enthalpy change
= enthalpy change
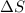 = entropy change
= entropy change
T = temperature in Kelvin
 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = -ve, reaction is spontaneous
= -ve, reaction is spontaneous
 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
Thus when
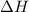 = -ve
= -ve
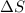 = +ve
= +ve
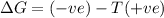
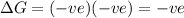
Reaction is spontaneous at all temperatures.