Answer:
C. 1,314,718 J
Step-by-step explanation:
The heat energy needed to raise the temperature of the coal is given by:
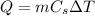
where:
m = 5 kg is the mass of the coal
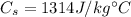 is the specific heat of coal
is the specific heat of coal
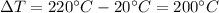 is the increase in temperature
is the increase in temperature
Substituting into the formula, we find
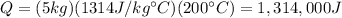
So, the closest option is
C. 1,314,718 J