Step-by-step explanation:
A catalyst helps in increasing the rate of a chemical reaction without itself getting consumed in the reaction.
Basically, a catalyst decreases the activation energy so that reactant molecules can easily participate in the reaction.
For example, when a catalyst is added to
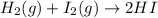 then there will be a decrease in activation energy and both reactants (hydrogen and iodine) can easily participate in the chemical reaction.
then there will be a decrease in activation energy and both reactants (hydrogen and iodine) can easily participate in the chemical reaction.
As a result, formation of product (HI) becomes faster.
Thus, we can conclude that a catalyst helps in increasing the rate of a reaction.