Answer:
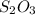
Step-by-step explanation:
Hello,
Nomenclature, is a powerful tool in chemistry to name chemical compounds to successfully distinguish among the thousands and thousands of existing molecular compounds. In rule to stipulate a chemical name y is via specifying the amount of the composing elements into the molecule, thus, the prefix di, accounts for two atoms and the prefix tri for three (this is related with the valence electrons the element has), in such a way, disulfur trioxide is represented with two sulfur atoms and three oxygen atoms, this is:
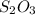
Which would be a novel molecule since the sulfur has been reported to have +2,+4 and +6 as the positive oxidation states which are possible to dovetail with oxygen as it is negative.
Kind regards.