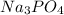Answer: The molecular formula for this compound is
Step-by-step explanation:
We are given a chemical compound known as sodium phosphate. It is formed by the interaction of oppositely charged ions.
The ions forming this compound are sodium ion
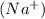 and phosphate ion
and phosphate ion
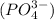
To know the chemical formula for a compound, we use criss-cross method, in which the charge of the ion becomes the subscript of the other ion.
Hence, the molecular formula for given compound will be