Answer: Polar molecules have an equal number of positive and negative particles.
Explanation: There are two types of molecules:
1) Polar molecules: This molecule is formed when two different atoms having different electronegativities combine together. The more electronegative atom attracts the shared pair of electron towards itself, thus acquiring a partial negative charge and other atom acquiring partial positive charge. This results in the formation of dipole. In this equal number of positive and negative particles.
For example: HCl. As this molecule is covalent in nature and is also a polar molecule.
2) Non-polar molecules: This is formed when two atoms of same electronegativity combine together. For Example:
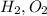 etc.
etc.
Hence, Polar molecules have an equal number of positive and negative particles.