Hello!
If 832J of energy is required to raise the temperature of a sample of aluminum from 20.0°C to 97.0°C, what mass is the sample of aluminum? (The specific heat of aluminum is 0.90 J/(g × °C).
a) 0.10 g
b) 10.0 g
c) 12.0 g
d) 57.7 g
Data:
Q (Amount of heat) = 832 J
m (mass) = ?
c (Specific heat) = 0.90 J/(g × ° C)
T (final) = 97 ºC
To (initial) = 20 ºC
ΔT = T - To → ΔT = 97 - 20 → ΔT = 77 ºC
Formula:
Q = m*c*ΔT
Solving:


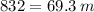
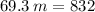
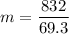

Answer:
12.0 g
____________________________________
I Hope this helps, greetings ... Dexteright02! =)