Answer : The concentration of
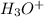 at equilibrium is, 0.015 M
at equilibrium is, 0.015 M
Solution :
The balanced equilibrium reaction will be,
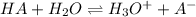
The expression for dissociation constant of weak aciod will be,
![k_a=([H_3O^+]* [A^-])/([HA])](https://img.qammunity.org/2018/formulas/chemistry/college/i7tgnlx47mr2nccyn0mfryk8njp823l1dh.png)
where,
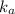 = dissociation constant of weak acid
= dissociation constant of weak acid
Let the concentration of
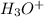 and
and
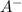 be 'x'
be 'x'
Now put all the given values in this expression, we get
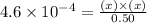
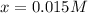
The concentration of
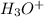 =
=
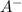 = x = 0.015 M
= x = 0.015 M
Therefore, the concentration of
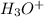 at equilibrium is, 0.015 M
at equilibrium is, 0.015 M