Answer: The correct answer is Option E.
Step-by-step explanation:
For the given options:
- Option A: Decomposition reaction
This reaction is defined as the chemical reaction in which a single substance breaks down into two or more simpler substances.
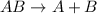
- Option B: Double displacement reaction
This reaction is defined as the chemical reaction in which exchange of ions takes place.
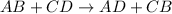
- Option C: Neutralization reaction
This reaction is defined as the chemical reaction in which an acid reacts with a base to produce a salt and water molecule.

- Option D: Single displacement reaction
This reaction is defined as the chemical reaction in which more reactive element displaces a less reactive element from its chemical reaction.
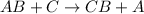
- Option E: Synthesis reaction
This reaction is defined as the chemical reaction in which substances combine in their elemental state to form a single compound.
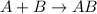
For the given chemical reaction:
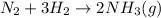
This reaction is considered as a synthesis reaction because hydrogen and nitrogen are combining in their elemental state to produce ammonia molecule.
Hence, the correct answer is Option E.