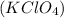Answer : The perchloric acid and potassium hydroxide base is used to prepare the salt of potassium perchlorate,
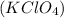
Explanation :
when the perchloric acid react with the potassium hydroxide as a base to form a salt of potassium perchlorate,
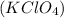
The balanced chemical reaction will be,
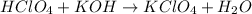
By the stoichiometry, we can say that 1 mole of perchloric acid react with the 1 mole of potassium hydroxide base to give 1 mole of potassium perchlorate and 1 mole of water as a product.
Hence, the perchloric acid and potassium hydroxide base is used to prepare the salt of potassium perchlorate,