Answer:The specific heat of the unknown substance is 1.22 J/ °Cg.
Step-by-step explanation:
Heat absorbed by the water .
Mass of the water = 110.0 g
Change in temperature of water =
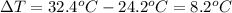
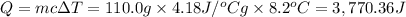
Heat lost by substance(Q') = Heat gained by the water(Q)
-Q' = Q
Change in temperature of the substance =
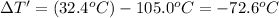
Mass of the substance = m'=42.5 g
Specific heat of substance = c'
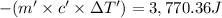
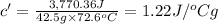
The specific heat of the unknown substance is 1.22 J/ °Cg.