Answer:
The chemical reaction is :
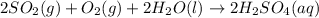
Step-by-step explanation:
When sulfur dioxide gas reacts with oxygen gas and liquid water it gives aqueous solution of sulfuric acid.
This is reason behind the acidic nature of the rain water during acid rain.The chemical reaction is given as:
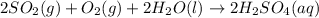
According to stoichiometry, 2 moles of sulfur dioxide gas reacts with 1 mole of oxygen gas and 2 moles of water to give 2 moles of aqueous sulfuric acid.