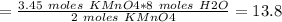Answer:
Moles of H2O produced = 13.8
Step-by-step explanation:
Given:
Moles of KMnO4 reacted = 3.45
To determine:
moles of H2O produced
Step-by-step explanation:
Given reaction:
2KMnO4 + 16HCl →2KCl + 2MnCl2 + 8H2O + 5Cl2
Based on the reaction stoichiometry:
2 moles of KMnO4 produces 8 moles of H2O
Therefore, moles of H2O produced when 3.45 moles of KMnO4 react is: