Answer:
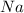
Step-by-step explanation:
Oxidation reaction : When there is a loss of electrons and thus an increase in oxidation number.
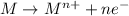
Reduction reaction : when there is a gain of electrons and thus a decrease in oxidation number.
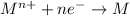
Sodium metal has gone under oxidation, as its oxidation state is changing from 0 in
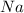 to +1 in
to +1 in
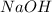
 ion has gone under reduction, as its oxidation state is changing from +1 in
ion has gone under reduction, as its oxidation state is changing from +1 in
 to 0 in
to 0 in
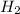
Those chemical agents which get oxidized itself and reduce others is called reducing agents. Thus
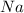 is a reducing agent here.
is a reducing agent here.