Hello!
We have an isobaric transformation, that is, when a certain mass under pressure maintains its constant pressure, on the other hand, as we increase the temperature, the volume increases and if we lower the temperature, the volume decreases and vice versa .
We have the following data:
V1 (initial volume) = 3.75 L
V2 (final volume) = 6.52 L
T1 (initial temperature) = 100 K
T2 (final temperature) =? (in Kelvin)
We apply the data to the formula of isobaric transformation (Gay-Lussac), let us see:
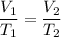
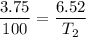

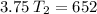
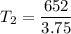
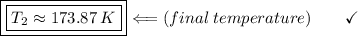
_________________________
I Hope this helps, greetings ... Dexteright02! =)