Answer:
The molality is
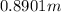
Step-by-step explanation:
Let's start defining the molality.
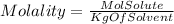
We also know that in terms of masses :
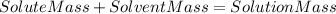 (I)
(I)
Finally, we define the mass percent as :
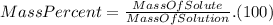
Using the data of the mass percent we find that :
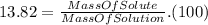
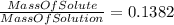 ⇒
⇒
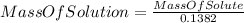 (II)
(II)
We know that the molar mass of glucose is
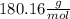
Therefore, if we use the mass of 1 mole of glucose (
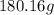 ) in (II) ⇒
) in (II) ⇒
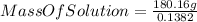
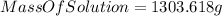
Now, if we use the equation (I) :
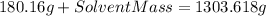
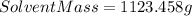
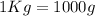 ⇒
⇒
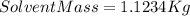
We find that 1 mole of glucose (
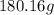 of glucose) are combined with
of glucose) are combined with
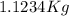 of solvent to obtain
of solvent to obtain
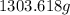 of solution which is a 13.82% by mass glucose solution.
of solution which is a 13.82% by mass glucose solution.
If we want to find the molality, we can replaced all the data in the equation of molality :
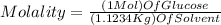
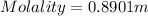
We use 1 mol of glucose in the equation (which corresponds to 180.16 g of glucose)
The letter ''m'' is the unit of molality.