Answer:
The amount of mass in kilograms would be lost
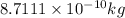 .
.
Step-by-step explanation:
Energy released during fusion reaction =
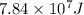
Mass of amount lost during the reaction = Δm
Speed of the light = c =
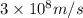
Using Einstein equation :
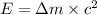
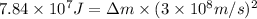
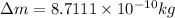
The amount of mass in kilograms would be lost
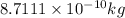 .
.