Answer: The element which is most likely to form negative ion is fluorine.
Step-by-step explanation:
An ion is formed when a neutral atom looses or gains electrons.
- When an atom looses electrons, it results in the formation of positive ion known as cation.
- When an atom gains electrons, it results in the formation of negative ion known as anion.
For the given options:
Aluminium is the 13th element of the periodic table having electronic configuration of
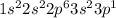
This element will loose 3 electrons to attain stable electronic configuration and will form
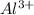 ion
ion
Copper is the 29th element of the periodic table having electronic configuration of
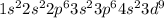
This element will loose 2 electrons to attain stable electronic configuration and will form
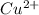 ion
ion
Sodium is the 11th element of the periodic table having electronic configuration of
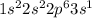
This element will loose 1 electron to attain stable electronic configuration and will form
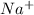 ion
ion
Fluorine is the 9th element of the periodic table having electronic configuration of
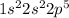
This element will gain 1 electron to attain stable electronic configuration and will form
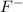 ion
ion
Hence, the element which is most likely to form negative ion is fluorine.