Answer:
The coefficient of the diatomic oxygen has to be 2.
Step-by-step explanation:
The balanced reaction should be like this:
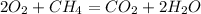
So, as you can see, balance mean that molecules in the reaction has to be equal, the amount of element involved has to be the same, due to the Conservation of the Mass Law.
So, Hydrogen and Oxygen are not balanced in the given expression, that's why we need to add those coefficients.