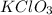To solve this problem, let us all convert the mass of each element into number of moles using the formula:
moles = mass / molar mass
Where,
molar mass K = 39.10 g / mol
molar mass Cl = 35.45 g / mol
molar mass O = 16 g / mol
and mass O = 13 g – 4.15 g – 3.76 g = 5.09 g
moles K = 4.15 g / (39.10 g / mol) = 0.106 mol
moles Cl = 3.76 g / (35.45 g / mol) = 0.106 mol
moles O = 5.09 g / (16 g / mol) = 0.318 mol
The ratio becomes:
0.106 K: 0.106 Cl: 0.318 O
We divide all numbers with the smallest number, in this case 0.106. This becomes:
K: Cl: 3O
Therefore the empirical formula is: