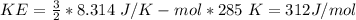Answer:
The average KE = 312 J/mol
Step-by-step explanation:
Given:
Temperature of the gas, T = 285 K
Gas constant, R = 8.314 J/K-mol
To determine:
The average kinetic energy of the gas
Step-by-step explanation:
Based on the kinetic theory of gases, the average kinetic energy is given as:
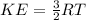
In this case: