Answer:
t = 45.5 million years
Step-by-step explanation:
Initially whole sample is consisting the radioisotope
so initial total mass will be
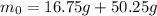
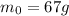
now after some time we can say the radioactive nuclei is of mass
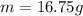
now we also know that half life is 23 million years
so we have
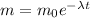
now we have
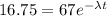
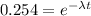
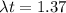
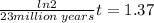
t = 45.5 million years
so above is the time interval