Answer : The reduction reaction takes place at cathode.
Explanation :
Voltaic cell : It is defined as a device which is used for the conversion of the chemical energy produces in a redox reaction into the electrical energy.
In the voltaic cell, the oxidation occurs at an anode which is a negative electrode and the reduction occurs at the cathode which is a positive electrode.
For example : The redox reaction occurs between the zinc and copper sulfate.
The balanced two-half reactions will be,
Oxidation half reaction (cathode) :
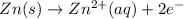
Reduction half reaction (anode) :

Thus the overall reaction will be,

Hence, the reduction reaction takes place at cathode.