Answer: No, the oxidation state of hydrogen does not change in the given chemical reaction.
Explanation: Balancing the given chemical equation, we get:
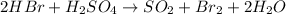
Reactant side:
In HBr, the oxidation state of bromine is -1, so to form a neutral compound, hydrogen should have an oxidation state of +1.
In
 , the oxidation state of sulfate is -2, so to form a neutral atom, two hydrogen atoms having +1 oxidation state should combine with it.
, the oxidation state of sulfate is -2, so to form a neutral atom, two hydrogen atoms having +1 oxidation state should combine with it.
Product side:
In
 , the oxidation state of hydroxide ion is -1, so to form a neutral water molecule, hydrogen should have an oxidation state of +1.
, the oxidation state of hydroxide ion is -1, so to form a neutral water molecule, hydrogen should have an oxidation state of +1.
From above, we can say that the oxidation states of hydrogen atom did not change for the following chemical reaction.