Step-by-step explanation:
When we obtain insoluble solid from a liquid solution, then this insoluble solid is known as precipitate.
When silver nitrate and potassium chromate solutions are mixed together then there is formation of silver chromate and potassium nitrate.
The reaction is as follows.
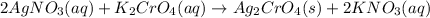
This insoluble solid
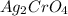 is the precipitate and it has a red-brown color.
is the precipitate and it has a red-brown color.