Answer : The correct option is, 2.0 M NaCl
Explanation :
Formula used for lowering in freezing point :
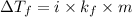
where,
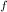 = change in freezing point or freezing point depression
= change in freezing point or freezing point depression
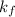 = freezing point constant
= freezing point constant
m = molality
i = Van't Hoff factor
As we know that the Van't Hoff factor for NaCl will be same for all given concentrations of NaCl and
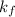 is the constant term. So, freezing point depression directly depends only on the molality of the solution.
is the constant term. So, freezing point depression directly depends only on the molality of the solution.
That means the more the value of molality, the lower will be the freezing point and vice-versa.
From the given options, 2.0 M NaCl has the lowest freezing point.
Hence, the correct option is, 2.0 M NaCl