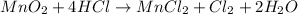Answer
Step-by-step explanation
The given unbalanced equation is:
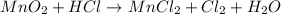
Step 1: Balance the Mn atom on both sides of the equation:
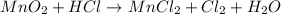
Step 2: Balance the Cl atom on both sides of the equation by putting 4 as the coefficient of HCl on the left side of the equation:
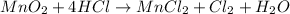
Step 3: Balance the O and H atoms by putting 2 as the coefficient of H₂O on the right side of the equation:
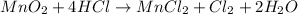
Hence, the balanced equation is: