Answer:
Molar concentration of ammonia gas is 0.0987 M.
Step-by-step explanation:
Concentration of hydrogen gas =
![[H_2]=0.15 M](https://img.qammunity.org/2018/formulas/chemistry/college/2xyufc4342hgsp7u7wyzzqnutgbllgerb5.png)
Concentration of nitrogen gas =
![[N_2]=0.017 M](https://img.qammunity.org/2018/formulas/chemistry/college/c0yhmfsecpazf68l6eyy5j7314rl1ibmsb.png)
Concentration of ammonia gas =
![[NH_3]=x](https://img.qammunity.org/2018/formulas/chemistry/college/eeks9awqp6gdrhm7ekvsa07leyq8359rof.png)
Equilibrium constant of the reaction =
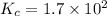
![K_c=([NH_3]^2)/([H_2]^3* [N_2])](https://img.qammunity.org/2018/formulas/chemistry/college/e41ztdozyiww2cd0vtkiu7j78zesltymru.png)
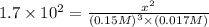
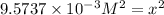
x = 0.0987 M
Molar concentration of ammonia gas is 0.0987 M.