Answer:
B) Nitrogen monoxide gas plus hydrogen gas yields ammonia gas plus water vapor
Step-by-step explanation:
The given reaction is:
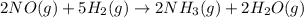
Here the chemical names of the reactants are:
NO = nitric oxide or nitrogen monoxide in gas phase
H2 = hydrogen gas
NH3 = ammonia in gas phase
H2O = water in gas phase i.e. vapor
Therefore the reaction can be expressed in words as:
Nitrogen monoxide gas plus hydrogen gas yields ammonia gas plus water vapor