Step-by-step explanation:
Alpha decay: In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
The general alpha decay reaction is given as:
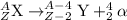
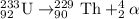
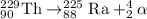
When Uranium-233 undergoes alpha decay it splits into an alpha particle and thorium-229.Thorium-229 further undergoes alpha decay to produce radium-225 along with an alpha particle.