Answer: Amount of heat required to vaporize 84.8 g of water is 191.7kJ.
Explanation: We are given 84.8 grams of water, to convert it into moles, we use the formula:
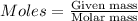
Molar mass of water = 18 g/mol
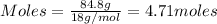
As we know that
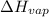 for 1 mole of water at 100° C is 40.7 kJ/mol
for 1 mole of water at 100° C is 40.7 kJ/mol
So, to calculate the amount of heat required, we use the formula:
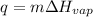
Putting the values in above equation, we get
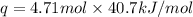
q = 191.7kJ