Answer: The given equation is not balanced in its lowest form because the coefficients could be reduced to 2, 1 and 3.
Explanation: We are given a equation but it is not balanced in its lowest form, so to reduce it into its lowest form, we divide the whole equation by 2.
The equation becomes:
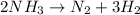
The coefficients for the equation are 2, 1 and 3.