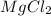Answer:
The correct formula for the compound made when aqueous solutions containing a dissolved Magnesium and Chloride are mixed is Magnesium chloride
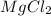
Step-by-step explanation:
If we suppose the existence of two components in aqueous solutions containing Magnesium and Chloride. For example: Hydrochloric acid - HCl and Magnesium hydroxide -
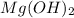 .
.
The global reaction would be:
HCl +
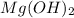 -->
-->
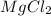 +
+
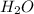
It means, Hydrochloric acid is neutralized with Magnesium hydroxide to produce Magnesium chloride and Water.
Besides of that, we can analyze the aqueos solution of every componenent:
For Hydrochloric acid:
HCl +
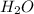 -->
-->
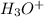 +
+
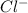
For Magnesium hydroxide
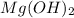 +
+
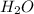 -->
-->
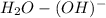 +
+
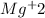
Finally, the ionic compounds will form the salt:
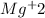 +
+
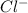 ->
->