This question requires understanding of oxidation states and differentiating between a reducing agent and an oxidizing agent. An oxidizing agent gains electrons and is reduced, which means the oxidation number decreases. A reducing agent loses electrons and is oxidized, which means an increase in oxidation state.
In the equation,
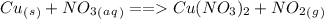
Cu in the solid state has 0 charge but in
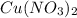 it loses 2 electrons to become
it loses 2 electrons to become
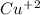 ion with a +2 charge, while nitrate
ion with a +2 charge, while nitrate
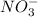 gains electrons to become nitrite
gains electrons to become nitrite
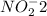 , -2 charge. Therefore we can conclude that Cu is the reducing agent.
, -2 charge. Therefore we can conclude that Cu is the reducing agent.