Answer: Partial pressure of ammonia gas will be 0.36 atm.
Step-by-step explanation: According to question we have partial pressure values of hydrogen and nitrogen gas with total pressure exerted by the all gases which hydrogen , nitrogen and ammonia. given
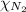 = 0.26 atm
= 0.26 atm
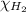 = 0.28 atm
= 0.28 atm
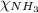 = ?
= ?
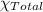 =0.90 atm
=0.90 atm
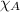 +
+
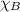 +
+
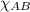 =
=
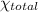 ..(1)
..(1)
putting values in (1)
0.26 + 0.28 +
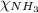 = 0.90
= 0.90
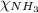 = 0.36 atm
= 0.36 atm